COVIQUICK CBNAAT Assay
Corona virus is a family of viruses generally responsible for causing respiratory and gastrointestinal illness. In 2019, a new Corona virus, Severe Acute Respiratory Syndrome Corona virus 2 emerged in China and later got spread across the globe as a pandemic. Respiratory disease caused by SARS-CoV-2 can manifest as an asymptomatic infection in some people and in symptomatic people, the symptoms can range from mild to moderate symptoms mimicking influenza like illness. In a minority group, the disease may manifest with more severe symptoms including pneumonia, shortness of breath etc. and require hospitalization.

COVID-19 disease can affect the upper respiratory tract and may even descend further to affect the lower respiratory tract including lungs. It is contagious and spreads in the same way through aerosols and fomites as other corona virus diseases like SARS and MERS. In order to combat the infection, gold standard test for its detection is Real time Reverse transcription PCR. The sensitivity, accuracy and user-friendliness of conducting this cartridge based test would enable it to be used at ports, stations, field and patient’s site etc. Robust and time-tested technologies: RT-PCR based Lab-on-Chips platform with the magnetic beads based nucleic acid extraction system involving minimal user interference makes this assay as a true point of care diagnostic system. Further, it yields excellent accuracy and sensitivity with a very quick sample to result turn-around time.
CoviQuick Cartridge Technical Specifications
Pathogen to be identified: SARS CoV-2
Assay: Taqman Based RT-PCR
Compatible equipment: Nobelza spectrophotometer
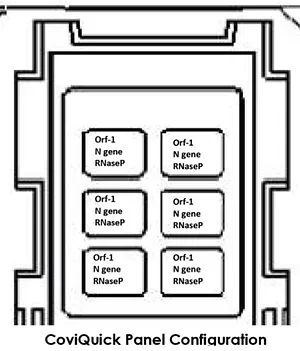
Number of reaction wells: Six
Number of patients to be tested per chip: Six
Nominal reaction volume per well: 5 µL
Cartridge Useability: One time usable (refer disposable guidelines)
Storage Condition: 2-8°C for six months
Shelf Life: 6 months
Medical Device Class: Class C
Manufacturer: InkJet Solutions Pvt. Ltd.
Validation
National Institute of Biologicals, Noida, a WHO collaborating center for quality control of in vitro diagnostics assays, tested and validated the kit.
 Brochure
Brochure