MTBQUICK CBNAAT Assay
Tuberculosis (TB) is still a major public health threat worldwide, with 10 million new cases and 1.2 million deaths every year. The risk of developing active TB following infection depends on age, the quality of the immune defense mechanisms, and the time elapsed since the infection. The estimated liftetime risk is 5-10%, but it is higher in small children, immunocompromised individuals, and shortly (within 1-2 years) after a contact with a contagious TB case.
Early and effective treatment is crucial to prevent the emergence of drug resistance strains. This demands the availability of fast and reliable point-of-care (POC) diagnostic methods for effective case management. Robust and time-tested technologies: RT-PCR based Lab-on-Chips platform with the magnetic beads based nucleic acid extraction system involving minimal user interference makes this assay as a true point of care diagnostic system. Further, it yields excellent accuracy and sensitivity with a very quick sample to result turn-around time..
MTBQuick Cartridge Technical Specifications
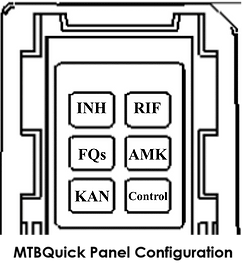
Antibiotic resistance can be identified: Isoniazid (INH), Rifampicin (RIF), Fluoroquinoline (FQs), Amikacin (AMK), Kanamycin (KAN)
Assay: Taqman Based RT-PCR
Compatible equipment: Nobelza spectrophotometer
Number of reaction wells: Six
Number of patients to be tested per chip: one
Nominal reaction volume per well: 5 µL
Cartridge Use-ability: One time usable (refer disposable guidelines)
Storage Condition: 2-8°C
Shelf Life: 6 months
Medical Device Class: Class C
 Brochure
Brochure
In support
Nobelza Point of Care (POC) diagnostic products have the potential to change the way doctors make diagnoses by bringing diagnostic capabilities closer to the patient. Our products are fast and accurate, making them ideal for use in remote or underserved areas, emergency situations, and even in-home settings. Our POC diagnostic product business model focuses on both the technological and commercial aspects of the product. The model is intended to ensure that the product is developed using cutting-edge technology while also being economically and commercially viable. Identifying a market need and developing a product to satisfy that need is the business model. Following that is product design and development, which includes the integration of necessary technology to guarantee efficiency and accuracy. We are looking for OEM partners to help us develop diagnostic assays or to manufacture, market, and sell these assays in conjunction with Nobelza real-time detection equipment as part of this model. We believe that an effective techno-commercial business model for a PoC diagnostic product requires a collaboration of technical expertise, market knowledge, and business acumen to guarantee that the product is both technologically advanced and financially viable. Please contact us if you share our enthusiasm for the development of novel diagnostics on our Nobelza platform.